The product: After identifying a gap in the market for a true regenerative medicine membrane, Orthocell developed CelGro™, a non-reactive resorbable membrane that promotes enhanced bone and soft tissue repair. This next-generation collagen-based scaffold is manufactured with Orthocell’s patented SMRT™ tissue engineering process. It is designed to produce a pure collagen scaffold and is devoid of reactive DNA, lipids and cell fragments, but preserves the collagen bundles and mechanical strength without crosslinking.

How it works: The barrier membrane’s exceptional regenerative properties are directly related to its composition and structure. The porous bilayer structure is bio-conductive, promoting cellular migration, adhesion and proliferation. Its pure Type I collagen bundles are osteoconductive and integrate to assist with remodeling the regenerating tissue into mature bone. The absorption rate in vivo provides optimal time for the formation of new bone while maintaining the barrier function to exclude the gingival epithelia.
Clinical results: In a case series of 20 patients who received guided bone regeneration (GBR) with CelGro™, investigators observed mature, corticalized bone with fully integrated graft material in all patients within three to five months (Fig. 1).
“CelGro®… enabled us to achieve outstanding guided bone regeneration, restoring both the quality and quantity of bone,” reported Dr. Brent Allan, Oral and Maxillofacial surgeon and chief investigator on the CelGro™ trial. “Our goal (with GBR) is to be able to restore normal anatomy, and … CelGro®… enabled us to achieve this goal with predictability.”
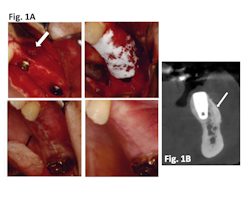
Figure 1A: Defect (arrow) with implant pre-GBR; CelGro™ in place over void filler and implant; wound closure at baseline; wound 4 weeks post-GBR. Images represented are from a single trial participant.
Figure 1B: CBCT scan after GBR with CelGro™ at 6 months in an individual trial participant. Increased density of medullary bone around implant fixture (arrow) is consistent with a healing response. Progressive consolidation and fusion of GBR to the buccal cortex on either side of the edentulous segment of the left mandible. Analyzed using DICOM software.
This predictability was so consistent the last 10 patients were treated using a single-stage procedure. No adverse events were reported at any stage during the trial.
These clinical results are supported by several users in Europe, who have given overwhelmingly positive feedback. They say CelGro™ is easy to handle, keeps its shape when placed over the bone graft, and stays in place without pins or sutures.
The advantages: CelGro™ outperforms market leaders in terms of its handling characteristics and ease of use and, most importantly, in the quality and quantity of bone regenerated. The pure collagen membrane is the only barrier membrane on the market developed by a company with more than 20 years of experience in regenerative medicine. The team’s understanding of the unique interaction between scaffold and cells was key to developing CelGro™ and its superior tissue regenerative capacity.
CelGro™ is not just a barrier membrane. It is a true regenerative medicine membrane designed by experts in regenerative medicine to deliver outstanding bone quantity and quality. The early maturation of bone means earlier crown placement, while predictable bone regeneration means single-stage implant techniques can be used with confidence to accelerate the healing process and improve both functional and esthetic outcomes for patients. CelGro™ is not an incremental improvement in GBR. CelGro™ is innovation at its best.
 By: The New Dentist
By: The New Dentist



